For adults with previously untreated Stage 3 or 4 classical Hodgkin lymphoma
How may I benefit from ADCETRIS?
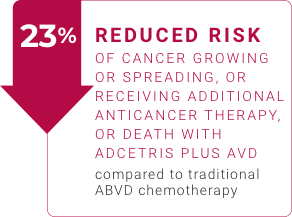
Before making decisions about your treatment, see how adults with classical Hodgkin lymphoma have responded to ADCETRIS.


*Amina was compensated by Pfizer for participating in this campaign.
The science of ADCETRIS treatment
ADCETRIS is not like traditional chemotherapy. It is an antibody drug conjugate made up of 3 parts: an antibody, a drug, and a linker.
Antibody
An antibody that finds CD30, a protein on the surface of certain cells. Antibodies are proteins made by the body’s immune system. The antibody that makes up ADCETRIS is made in a laboratory.
Drug
A drug that is designed to cause cell death.
Linker
A linker that attaches the drug to the antibody and is designed to release the drug inside the cell.
How does ADCETRIS work?
Step 1
ADCETRIS aims to attach to cells that have a protein on their surface called CD30.
Step 2
Once attached, ADCETRIS is brought into the cell and released.
Step 3
The drug stops the cell from being able to grow and divide, causing the cell to die.
CD30 is found on classical Hodgkin lymphoma cells and not commonly found on healthy cells. Even though ADCETRIS is a CD30-directed therapy, it can still harm normal cells and cause side effects. Talk to your doctor if you have questions about possible side effects.