For adults with previously untreated Stage 3 or 4 classical Hodgkin lymphoma
How may I benefit from ADCETRIS?
Before making decisions about your treatment, see how adults with classical Hodgkin lymphoma have responded to ADCETRIS.


*Amina was compensated by Pfizer for participating in this campaign.
Select each tab to see ADCETRIS study results for people with certain types of classical Hodgkin lymphoma
Previously untreated
Relapsed
After stem cell transplant
Previously untreated classical Hodgkin lymphoma
Relapsed classical Hodgkin lymphoma
After stem cell transplant
Study results
Side effects
What is the most important serious safety information I should know about ADCETRIS?
PROGRESSIVE MULTIFOCAL LEUKOENCEPHALOPATHY (PML): Patients treated with ADCETRIS can have a rare, serious brain infection called PML that can lead to death. Tell your doctor immediately if you have mood or behavior changes, confusion, problems in thinking or loss of memory, changes in vision, speech, or walking, or decreased strength or weakness on one side of the body. PML may also be caused by prior treatments or diseases that weakened your immune system.
Do not take ADCETRIS with bleomycin because of possible serious side effects to the lungs. These are not the only side effects of ADCETRIS. Always tell your doctor about any side effects you experience.
Most common side effects
In people treated with ADCETRIS alone, the most common side effects that occurred in ≥20% of adult patients were nerve damage (peripheral neuropathy), feeling tired, nausea, diarrhea, a low white blood cell count, upper respiratory tract infection, and fever.
In this study, the most common serious side effects were:
- Pneumonia (4%)
- Fever (4%)
- Vomiting (3%)
- Nausea (2%)
- Liver damage (2%)
- Peripheral sensory neuropathy (tingling or numbness in the hands or feet)
For more information on side effects, please see the Important Safety Information at the bottom of this page and read the Important Facts about ADCETRIS including IMPORTANT WARNING.
Most common side effects
In people treated with ADCETRIS plus AVD chemotherapy, the most common side effects that occurred in ≥20% of study patients were nerve damage (peripheral neuropathy), a low white blood cell count, nausea, constipation, vomiting, feeling tired, diarrhea, fever, hair loss, weight loss, stomach pain, a low red blood cell count, and sores or swelling in the mouth.
In this study, the most common serious side effects were:
- Significantly low numbers of white blood cells with a fever (17%)
- Fever (7%)
- Low numbers of white blood cells (3%)
- Pneumonia (3%)
For more information on side effects, please see the Important Safety Information at the bottom of this page and read the Important Facts about ADCETRIS including IMPORTANT WARNING.
Most common side effects
In people treated with ADCETRIS alone, the most common side effects that occurred in ≥20% of adult patients were nerve damage (peripheral neuropathy), feeling tired, nausea, diarrhea, a low white blood cell count, upper respiratory tract infection, and fever.
In this study, the most common serious side effects were:
- Peripheral motor neuropathy (weakness in the hands or feet) (4%)
- Stomach pain (3%)
- Blood clots in the lungs (2%)
- Inflamed lungs (2%)
- Collapsed lung (2%)
- Kidney infections (2%)
- Fever (2%)
For more information on side effects, please see the Important Safety Information at the bottom of this page and read the Important Facts about ADCETRIS including IMPORTANT WARNING.
Tell your doctor about any side effect concerns you have
Your doctor should prescribe granulocyte colony-stimulating factor (G-CSF) along with your ADCETRIS treatment right at the start. G-CSF is a medication that may help reduce the chance of neutropenia (low white blood cell count).
Don’t stop, change, or delay your ADCETRIS plus AVD treatment unless directed by your doctor. Your doctor may take additional steps to help manage side effects, including:
Don’t stop, change, or delay your ADCETRIS treatment unless directed by your doctor. Your doctor may take additional steps to help manage side effects, including:
- Reducing your ADCETRIS dosage, or delaying your next dose, until symptoms improve
- Stopping ADCETRIS completely if side effects are severe or do not improve
Glossary
Anemia: Not having enough red blood cells, which could make you feel tired or out of breath, or could make you need a blood transfusion.
G-CSF: Granulocyte colony-stimulating factor, a medication that can help boost white blood cell count.
Neutropenia: Having low levels of a type of white blood cell called neutrophils that help your immune system. You could have a higher chance of getting an infection.
Peripheral neuropathy: Nerve damage that can cause numbness or tingling in the hands or feet (sensory) and/or weakness in the arms or legs (motor).
Peripheral neuropathy: Nerve damage that can cause numbness or tingling in the hands or feet (sensory) and/or weakness in the arms or legs (motor).
Study design information
An international, clinical study of 102 people evaluated the effectiveness and safety of ADCETRIS in patients with classical Hodgkin lymphoma that came back after stem cell transplant.
- 102 people were assigned to receive ADCETRIS every 3 weeks for up to 16 cycles
Researchers reviewed the results of this treatment at approximately 1.5 years.
Select the button above to view side effects.
Study results
APPROXIMATELY 1.5 YEARS AFTER TREATMENT
ADCETRIS was an effective treatment for classical Hodgkin lymphoma that came back after stem cell transplant
- The following data represent the overall response rate (complete remission plus partial remission) that was observed at approximately 1.5 years
- These data are the primary endpoint in the study, and the results were used to help support the FDA approval of ADCETRIS
- The follow-up time was ongoing at the time of data submission
IN MOST ADCETRIS PATIENTS, THEIR RECURRING CLASSICAL
HODGKIN LYMPHOMA
BECAME REDUCED OR UNDETECTABLE
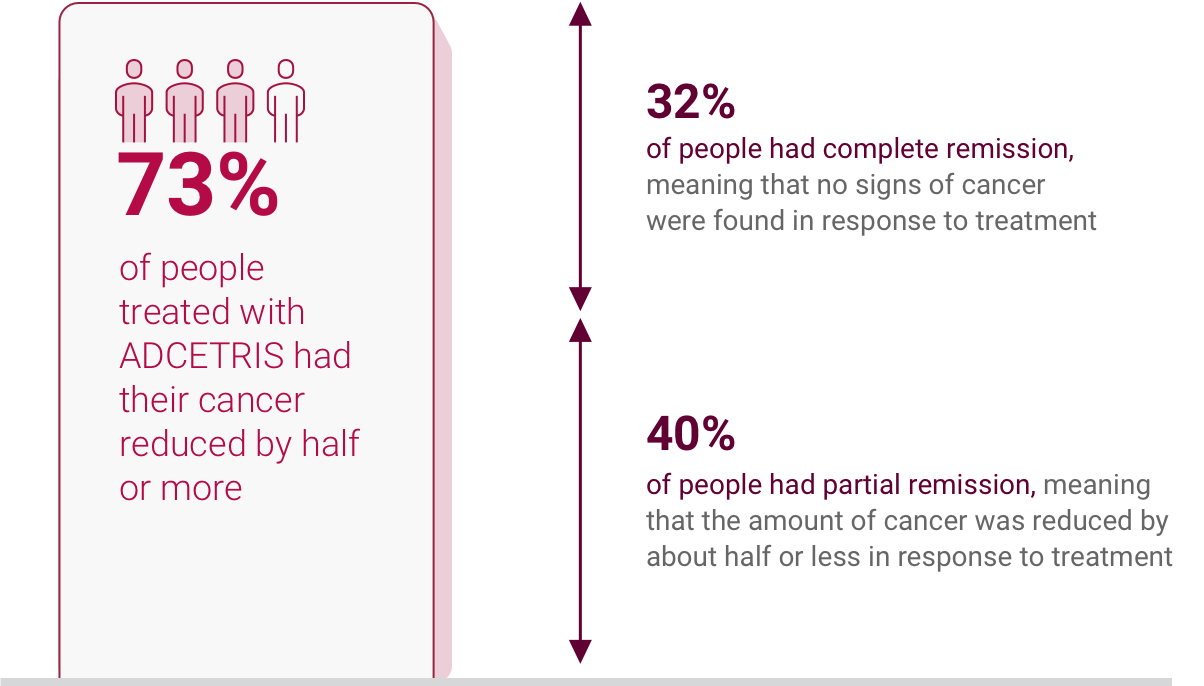
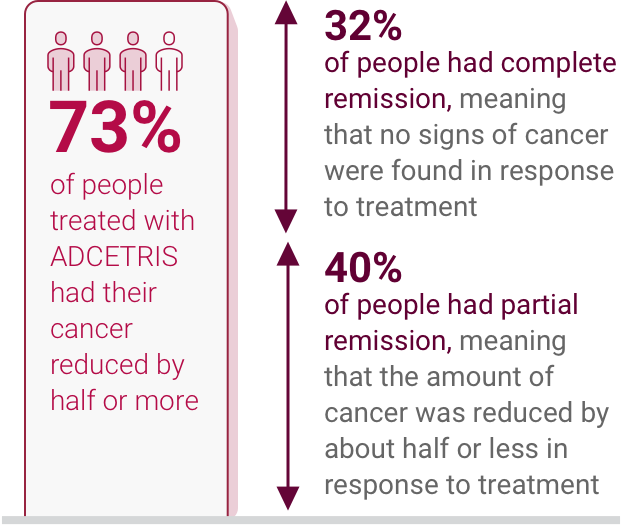
In the relapsed classical Hodgkin lymphoma
study, response to ADCETRIS treatment
lasted on
average
- 20.5 months in people with complete remission
- 3.5 months in people with partial remission
- 20.5 months in people with complete remission
- 3.5 months in people with partial remission
Tell your doctor about any side effect concerns you have
Don’t stop, change, or delay your ADCETRIS treatment unless directed by your doctor. Your doctor may take additional steps to help manage side effects, including:
- Reducing your ADCETRIS dosage, or delaying your next dose, until symptoms improve
- Stopping ADCETRIS completely if side effects are severe or do not improve
Glossary
Complete remission: When cancer can't be found in your body after treatment.
Partial remission: When some of the cancer has gone away after treatment but there is still some left.
AETHERA study design information
AETHERA was a large, clinical study of 329 people that compared the effectiveness and safety of ADCETRIS with placebo in patients with classical Hodgkin lymphoma that has a high risk of coming back or getting worse after a stem cell transplant.
- 165 people were assigned to receive ADCETRIS every 3 weeks for up to 16 cycles
- 164 people were assigned to receive placebo
High-risk classical Hodgkin lymphoma was defined in the study as when the first therapy does not work and the cancer does not respond to treatment, when disease returns within 12 months of the first treatment, or when disease returns more than 12 months after the first treatment, and there are signs of lymphoma in areas or organs outside the lymph nodes. Researchers reviewed the results of this treatment at approximately 2 years.
APPROXIMATELY 2 YEARS AFTER TREATMENT
ADCETRIS was an effective treatment for classical Hodgkin lymphoma that has a high risk of coming back or getting worse after a stem cell transplant
- The following data represent the progression-free survival that was observed at approximately 2 years
- Progression-free survival means the length of time from the start of treatment a patient lives without cancer progression or death
- These data are the primary endpoint in the study, and the results were used to help support the FDA approval of ADCETRIS
- The median follow-up time was approximately 2 years (22 months)
ADCETRIS was more effective than placebo
Compared to those receiving placebo, people treated with ADCETRIS on the study
- Experienced fewer disease progression events (36% of people receiving ADCETRIS compared with 46% receiving placebo)
- Had more time without disease progression
Length of time without cancer progression or death after the start of the study†, ‡:
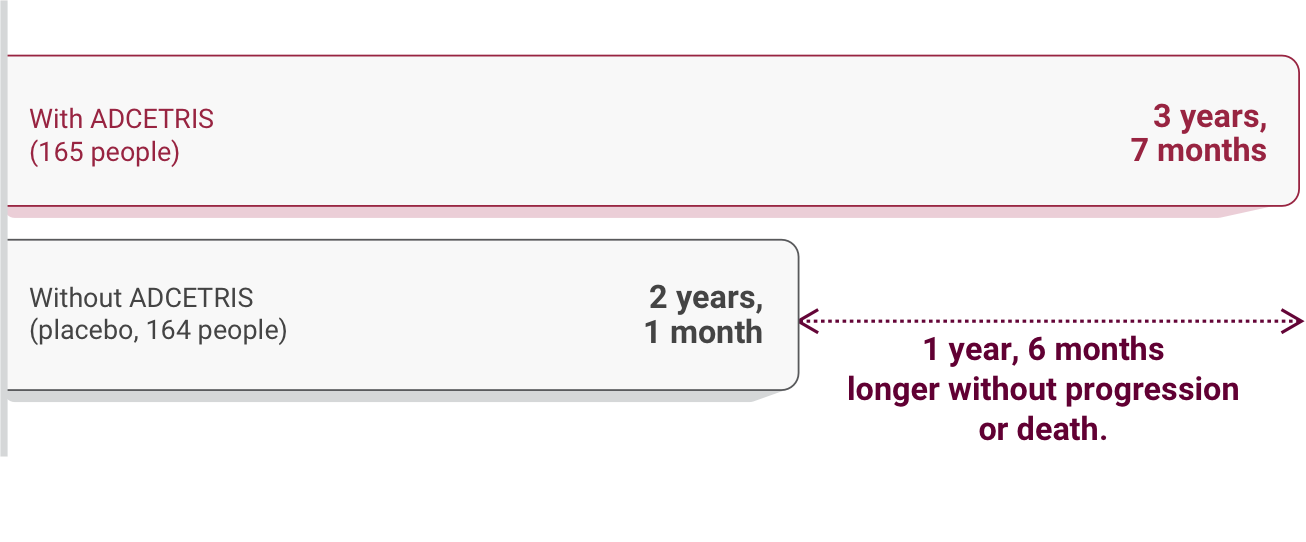
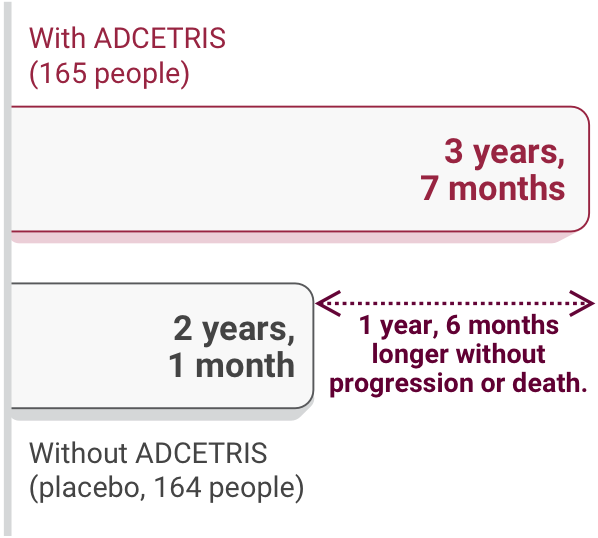
These lengths of time are median numbers.
†The median follow-up time was 22 months.
‡Lengths of time were rounded to the nearest whole month.
Tell your doctor about any side effect concerns you have
Don’t stop, change, or delay your ADCETRIS plus AVD treatment unless directed by your doctor. Your doctor may take additional steps to help manage side effects, including:
- Reducing your ADCETRIS dosage, or delaying your next dose, until symptoms improve
- Stopping ADCETRIS completely if side effects are severe or do not improve
Glossary
Median time: The time at which half of the patients experienced relapse or progression and half had not.
Placebo: A substance that does not contain medication and has no effect. In clinical studies, it looks the same and is given the same way as the medication being tested.
ECHELON-1 study design information
ECHELON-1 was a large, international, clinical study of 1334 people that compared the effectiveness and safety of ADCETRIS plus AVD with ABVD chemotherapy in patients with previously untreated Stage 3 and 4 classical Hodgkin lymphoma.
- 664 people were assigned to receive ADCETRIS plus AVD chemotherapy every 2 weeks for up to 12 doses
- 670 people were assigned to receive ABVD chemotherapy every 2 weeks for up to 6 cycles
Researchers reviewed the results of these treatments at approximately 2 years and collected data about long-term results at 6 years.
Select the button above to view side effects.
Study results
APPROXIMATELY 6 YEARS AFTER TREATMENT
Overall survival rate was higher with ADCETRIS plus
AVD
compared to ABVD
chemotherapy
- The following data represent the overall survival that was observed at 6 years
- Overall survival is the length of time, from the start of treatment, that patients are still alive
- These data are the secondary endpoint in the study
- The median follow-up time was 6.1 years


- About 94% of adults treated with ADCETRIS plus AVD, and about 89% of adults treated with ABVD, were still alive at 6 years
After 2 years of follow up
ADCETRIS plus AVD was more effective than ABVD
- The following data represent the modified progression-free survival that was observed at 2 years
- Modified progression-free survival means the length of time during and after treatment a patient lives without cancer progression, death, or receiving another cancer treatment
- These data are the primary endpoint in the study, and these results were used to help support the FDA approval of ADCETRIS plus AVD
- The median follow-up time was just over 2 years (24.6 months)

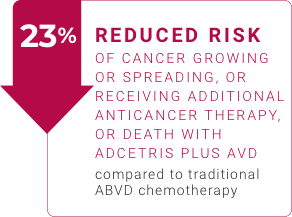
- About 82% of adults treated with ADCETRIS plus AVD did not have their cancer grow or spread, need additional anticancer therapy, or die compared to about 77% of adults treated with traditional ABVD chemotherapy
For additional 6-year follow-up data, review the Treatment Decision Guide, which can help you feel confident discussing options with your doctor.
Tell your doctor about any side effect concerns you have
Your doctor should prescribe granulocyte colony-stimulating factor (G-CSF) along with your ADCETRIS treatment right at the start. G-CSF is a medication that may help reduce the chance of neutropenia (low white blood cell count).
Don’t stop, change, or delay your ADCETRIS plus AVD treatment unless directed by your doctor. Your doctor may take additional steps to help manage side effects, including:
- Reducing your ADCETRIS dosage, or delaying your next dose, until symptoms improve
- Stopping ADCETRIS completely if side effects are severe or do not improve
Glossary
ABVD: A combination of 4 chemotherapies—Adriamycin, bleomycin, vinblastine, and dacarbazine.
AVD: A combination of 3 chemotherapies—Adriamycin, vinblastine, and dacarbazine.
FDA: Food and Drug Administration.
G-CSF: Granulocyte colony-stimulating factor, a medication that can help boost white blood cell count.
Median: The middle number in a list of numbers.
Neutropenia: Having low levels of a type of white blood cell called neutrophils that help your immune system. You could have a higher chance of getting an infection.
Overall survival: The length of time that patients remain alive after starting study treatment.
Amina’s treatment journey
aminaAmina knew something was wrong and spoke up. Listening to herself and her body is what helped Amina find out she had Stage 3 classical Hodgkin lymphoma. Throughout this challenging diagnosis, she found support and encouragement from her family, friends, and healthcare team.
ADCETRIS will not work for everyone.
The science of ADCETRIS treatment
ADCETRIS is not like traditional chemotherapy. It is an antibody drug conjugate made up of 3 parts: an antibody, a drug, and a linker.
Antibody
An antibody that finds CD30, a protein on the surface of certain cells. Antibodies are proteins made by the body’s immune system. The antibody that makes up ADCETRIS is made in a laboratory.
Drug
A drug that is designed to cause cell death.
Linker
A linker that attaches the drug to the antibody and is designed to release the drug inside the cell.
How does ADCETRIS work?
Step 1
ADCETRIS aims to attach to cells that have a protein on their surface called CD30.
Step 2
Once attached, ADCETRIS is brought into the cell and released.
Step 3
The drug stops the cell from being able to grow and divide, causing the cell to die.
CD30 is found on classical Hodgkin lymphoma cells and not commonly found on healthy cells. Even though ADCETRIS is a CD30-directed therapy, it can still harm normal cells and cause side effects. Talk to your doctor if you have questions about possible side effects.
