Take action in the treatment of certain peripheral T-cell lymphomas with ADCETRIS
ADCETRIS is approved to treat certain types of peripheral T-cell lymphomas that express the CD30 protein. Before making treatment decisions, learn more about the benefits and risks of ADCETRIS, and see how others responded.
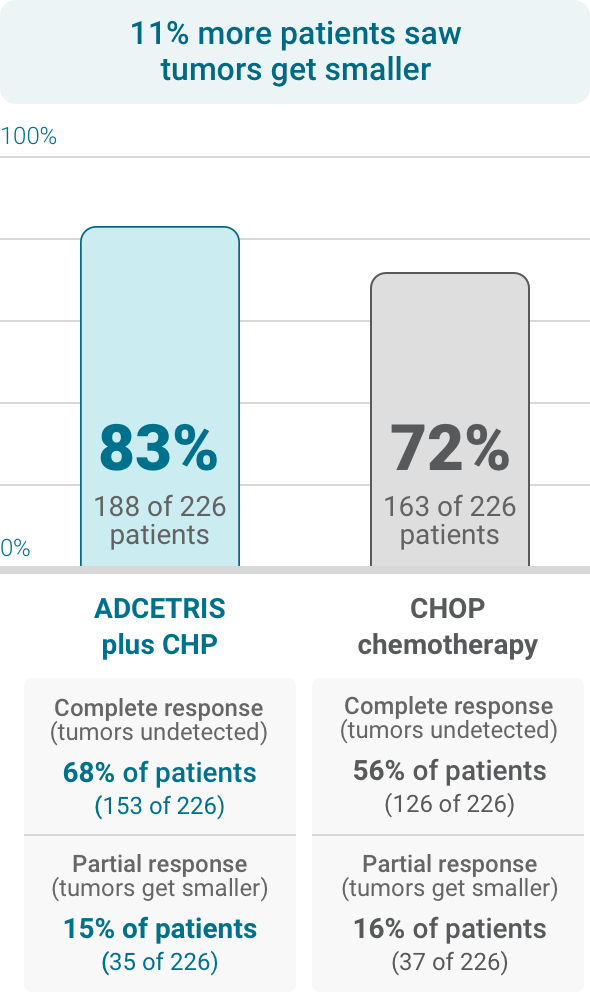
See study resultsHow does ADCETRIS work?
Step 1
ADCETRIS aims to attach to cells that have a protein on their surface called CD30.
Step 2
Once attached, ADCETRIS is brought into the cell and released.
Step 3
The drug stops the cell from being able to grow and divide, causing the cell to die.
CD30 is found on cancer cells of peripheral T-cell lymphomas, including these types:
- Adult T-cell lymphoma/leukemia
- Angioimmunoblastic T-cell lymphoma
- Enteropathy-associated T-cell lymphoma
- Peripheral T-cell lymphoma not otherwise specified
- Cutaneous T-cell lymphomas, including
mycosis fungoides - Anaplastic large cell lymphoma (ALCL), including systemic ALCL and
primary cutaneous anaplastic large cell lymphoma (pcALCL)
CD30 is found on peripheral T-cell lymphoma cells and not commonly found on healthy cells. Even though ADCETRIS is a CD30‑directed therapy, it may still harm normal cells and cause side effects. Talk to your doctor if you have questions about side effects.
Resources and support
From cost to resources, support may be available
The patient support programs available through Pfizer are designed to help patients begin their prescribed ADCETRIS treatment. If eligible and enrolled, you can receive personalized support, including:
- Confirming your insurance coverage
- Evaluating out-of-pocket costs and available copay options
- Helping you access alternative support options if you can’t afford ADCETRIS*
Talk to your healthcare provider to learn how to enroll in the Pfizer Patient Assistance Program.
Visit the websiteInformation provided by patient support programs from Pfizer is not intended to be a substitute for your healthcare provider. Discuss any questions you may have about your disease and your treatment with your healthcare team.
*Financial support may be provided through foundation referral. Pfizer does not guarantee that enrollment will result in coverage and/or reimbursement.
Apps to manage your care†
Focus on Lymphoma
This app from the Lymphoma Research Foundation offers:
- Tailored content for your type of cancer
- A way to track your medications, blood counts, and doctor discussions
- Educational resources, stories of hope, and diagnosis and treatment information
- Resources, financial assistance, counseling, and help finding a doctor
- Ways to connect with others
LLS Health Manager
This app from the Leukemia and Lymphoma Society features:
- Side effect, medication, food, and hydration trackers
- Questions for your doctor
- Meal-planning tools
- Shared caregiver access
- Education, support, and resources
Helpful links†
Connect with other people living with cancer, and their caregivers

Provides education, support, and advocacy resources for people with cutaneous lymphoma.

Hosts educational forums and provides online literature for people with T-cell leukemias and lymphomas, and their families.

Dedicated to funding research, finding cures, and ensuring access to treatments for patients. The Leukemia & Lymphoma Society (LLS) is committed to providing information, resources, and support to those affected by blood cancers.

Devoted to improving care through patient education and support services and improving outcomes through investment in the most promising lymphoma research.

Provides case management, counseling, support groups, education, publications, and financial and co-payment assistance.

Offers a global network of support that is available online and at community-based centers and hospitals.

Supports people with cancer who are trying to manage their career with expert advice, interactive tools, and educational events.

Provides transplant patients, survivors, and their loved ones with emotional support and information about bone marrow, peripheral blood stem cell, and cord blood transplants.
†Pfizer is not responsible for the content or services of these providers. The information/links provided by Pfizer are not meant to replace a physician's medical advice.
Glossary
CHOP: A combination of 4 chemotherapies—cyclophosphamide, doxorubicin, vincristine, and prednisone.
CHP: A combination of 3 chemotherapies—cyclophosphamide, doxorubicin, and prednisone.
Complete remission: When all signs of cancer in your body are undetected following treatment.
FDA: Food and Drug Administration.
G-CSF: Granulocyte colony-stimulating factor, a medication that can help boost white blood cell count.
Lymphoma: A type of blood cancer that starts in the cells of the immune system (or lymphatic system).
Median: The middle number in a list of numbers.
Neutropenia: Having low levels of a type of white blood cell called neutrophils that help your immune system. You could have a higher chance of getting an infection.
Partial remission: A decrease in the size of a tumor, or the amount of cancer in your body, following treatment. Some cancer remains.
Primary cutaneous anaplastic large cell lymphoma (pcALCL) and mycosis fungoides (MF): 2 different types of cutaneous T-cell lymphoma, a type of blood cancer that involves the skin. Mycosis fungoides is the most common type of cutaneous T-cell lymphoma, occurring in about 50% of cases. Primary cutaneous anaplastic large cell lymphoma happens less often.
Relapse: When cancer has returned after a period of remission.
Remission: When signs and symptoms of cancer are reduced or undetectable.
Systemic anaplastic large cell lymphoma (sALCL): A fast-growing type of T-cell lymphoma. It may appear in the skin, lymph nodes, bones, soft tissues, lungs, or liver. Its cancer cells have CD30 protein on them. Anaplastic large cell lymphomas comprise about 1% of all non-Hodgkin lymphomas and about 10-20% of all T-cell lymphomas.